![Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution. Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution.](https://haygot.s3.amazonaws.com/questions/2015518_249889_ans_bbd2828107e04c46b63fdcb0f0c04801.jpg)
Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution.
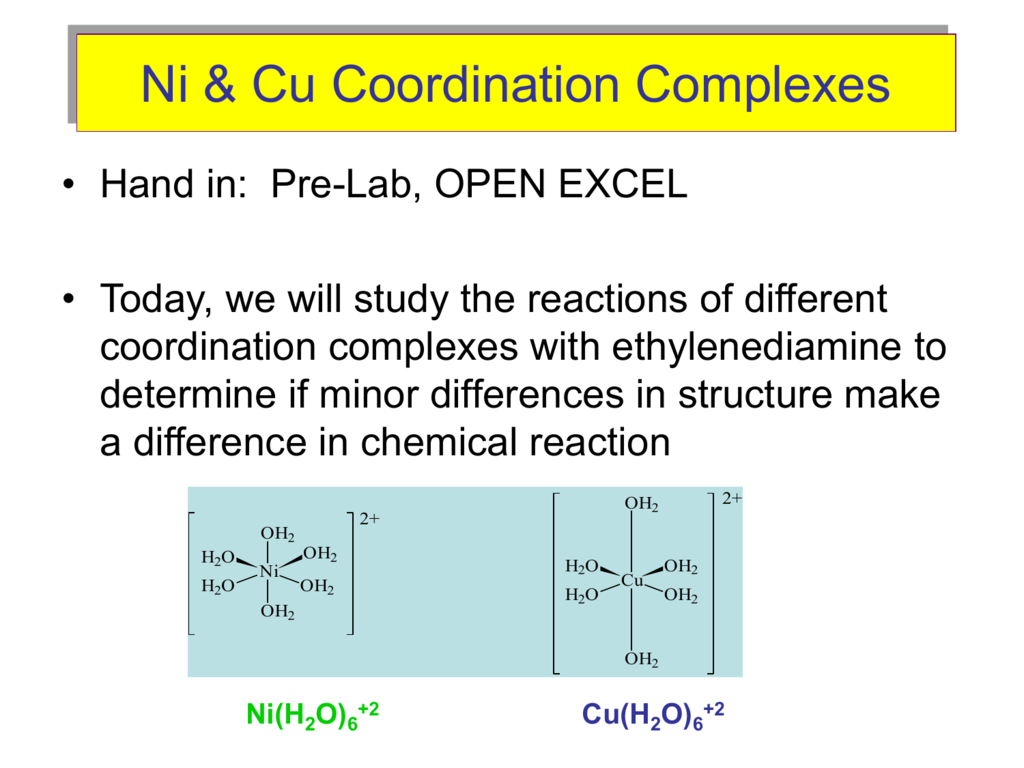
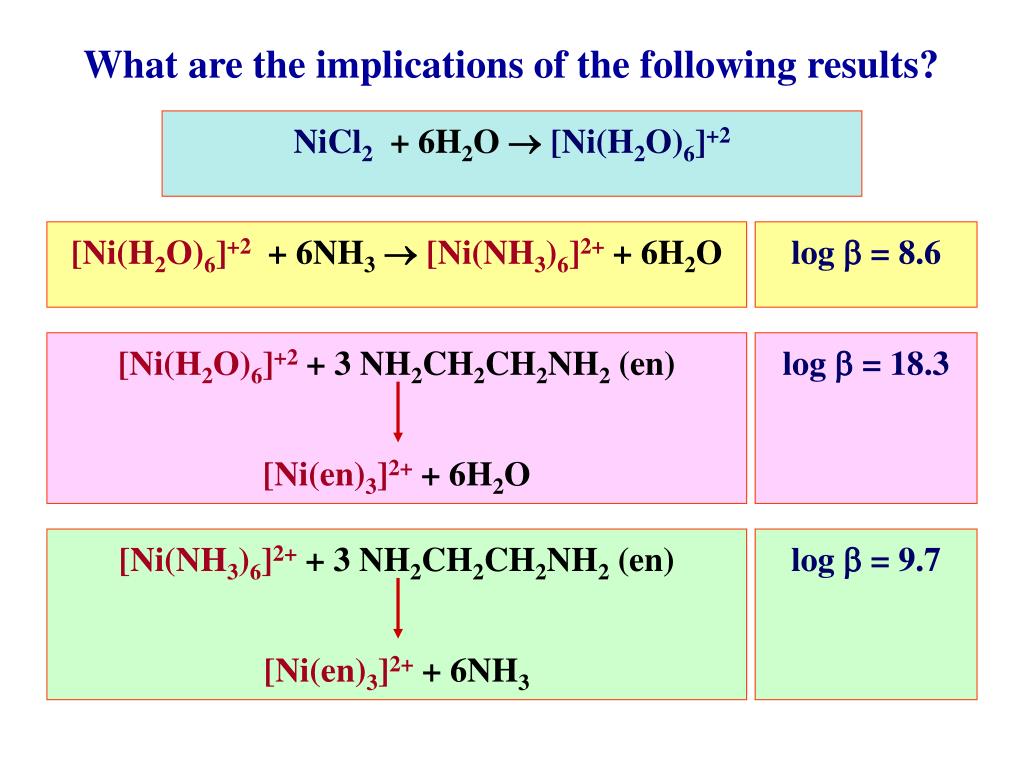
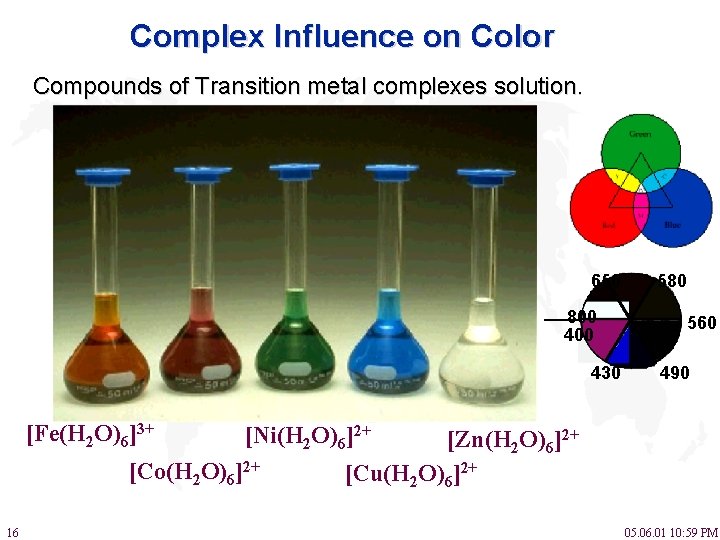
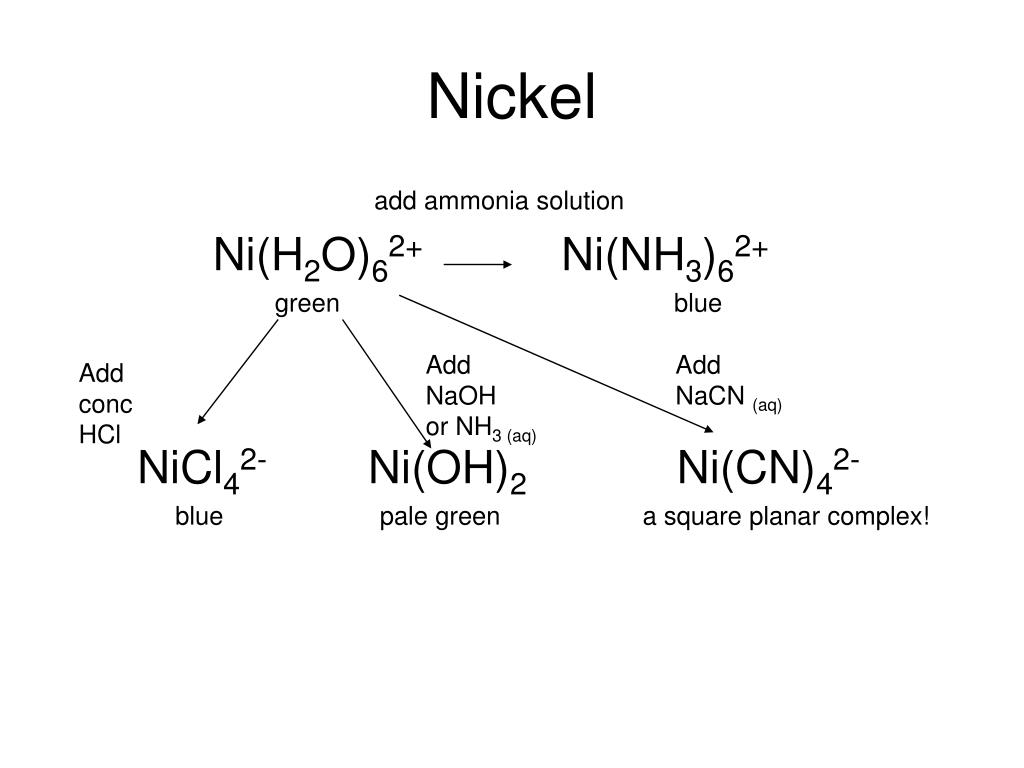
Lecture 6 Model Answers to Problems 1 Self-Study Problems / Exam Preparation • Explain the colour changes observed for the nic
![IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2 IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2](https://journals.iucr.org/e/issues/2020/03/00/xi2016/xi2016scheme1.gif)
IUCr) Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6 ](SO4)2
The hybridizations of Ni(CO)4 and Cr(H2O)6^2+, respectively, are - Sarthaks eConnect | Largest Online Education Community
![Triplet Electronic States in d2 and d8 Complexes Probed by Absorption Spectroscopy: A CASSCF/CASPT2 Analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+ | Inorganic Chemistry Triplet Electronic States in d2 and d8 Complexes Probed by Absorption Spectroscopy: A CASSCF/CASPT2 Analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+ | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/ic0010860/asset/images/medium/ic0010860n00001.gif)
Triplet Electronic States in d2 and d8 Complexes Probed by Absorption Spectroscopy: A CASSCF/CASPT2 Analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+ | Inorganic Chemistry
![A solution of [Ni(H2O)6]^(2+) is green, whereas a solution of [Ni (CN)4]^(2-) is colorless - Explain A solution of [Ni(H2O)6]^(2+) is green, whereas a solution of [Ni (CN)4]^(2-) is colorless - Explain](https://doubtnut-static.s.llnwi.net/static/web-thumb/645097983_web.png)
A solution of [Ni(H2O)6]^(2+) is green, whereas a solution of [Ni (CN)4]^(2-) is colorless - Explain
![A question from my textbook: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. Explain. : r/chemhelp A question from my textbook: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. Explain. : r/chemhelp](https://external-preview.redd.it/VDYxIhyF0tX1EvHO09Izi030EWZe0sji-zLW3q5xn38.jpg?auto=webp&s=05f1b2c012b7b91e46340d66d18806bcaa6e97b2)
A question from my textbook: A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. Explain. : r/chemhelp
![Assertion A solution of `[Ni(H_(2)O)_(6)]^(2+)` is green but a solution of `[Ni(CN)_(4)]^(2+)` is - YouTube Assertion A solution of `[Ni(H_(2)O)_(6)]^(2+)` is green but a solution of `[Ni(CN)_(4)]^(2+)` is - YouTube](https://i.ytimg.com/vi/nIpwGgykAQU/maxresdefault.jpg)
Assertion A solution of `[Ni(H_(2)O)_(6)]^(2+)` is green but a solution of `[Ni(CN)_(4)]^(2+)` is - YouTube
![Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_599e6db7e641a.png)




![Solved 20. When H2O in the [Ni(H2O)6]2+ complex ion is | Chegg.com Solved 20. When H2O in the [Ni(H2O)6]2+ complex ion is | Chegg.com](https://media.cheggcdn.com/media%2F782%2F7824ed9d-14a5-4257-bc8c-05dc5a9cf139%2FphpvCnudZ.png)